
Zonisamide
Transformed
An innovative approach to the molecule you know and trust
Azurity Pharmaceuticals, Inc. is thrilled to support the work of epilepsy communities, helping to make a meaningful impact for patients and their caregivers/families regardless of what medication they’re prescribed.
When you select a support or advocacy organization from this list, Azurity will contribute a $25 donation to further its mission.
Thank you for standing with us in support!

(NDC 52652-8001-1)
Product labeling, packaging, and imagery are for representation purposes only.
ZONISADE® (zonisamide oral suspension), 100 mg/5 mL is indicated as adjunctive therapy for the treatment of partial-onset seizures in adults and pediatric patients 16 years of age and older.
The ready-to-use* oral liquid formulation of ZONISADE® eliminates the need for compounding and crushing.
*Shake well before administering.
ZONISADE® can be stored at room temperature and should be protected from light.2
The beyond-use date of ZONISADE® is 30 days after a bottle is first opened.2
Administration and Storage
ZONISADE® is an oral liquid suspension and should be shaken well before every administration.
To administer directly into the mouth, it is important that ZONISADE® be measured with an accurate measuring device. Inform patients that a pharmacist will provide an appropriate device and instructions for measuring the correct dose. A household teaspoon is not an accurate measuring device.
ZONISADE® should be administered once or twice daily with or without food.
No refrigeration is required, as ZONISADE® can be stored at room temperature. It should also be protected from light. Once opened, unused portions should be discarded after 30 days.2
Dosing
The recommended initial dose of ZONISADE® is 100 mg daily. The dosage may be increased by 100 mg daily every 2 weeks to 400 mg daily based on clinical response and tolerability. However, evidence from controlled trials shows no suggestion of increasing response above 400 mg/day. Patients who are tolerating ZONISADE® at 400 mg daily and require further reduction of seizures may be increased up to a maximum dosage of 600 mg daily.
ZONISADE® has a prolonged half-life of 105 hours in red blood cells, which allows for a once-daily dosing regimen and results in steady therapeutic drug levels. This means that if a patient misses a dose, they can resume dosing at the regular schedule without a makeup dose.2
| Initial dose | 100 mg daily |
|---|---|
| Beyond 2 weeks | May be increased by 100 mg/day every 2 weeks to 400 mg/day†,‡ |
*Measure with an accurate measuring device. A household teaspoon is not an accurate measuring device.
†Based on clinical response and tolerability.
‡Patients who are tolerating 400 mg/day and require further reduction of seizures may be increased up to a maximum dosage of 600 mg/day. However, evidence from controlled trials shows no suggestion of increasing response above 400 mg/day.
Safety and efficacy in pediatric patients below 16 years of age have not been established. In patients with renal impairment, slower titration and more frequent monitoring may be required.
Conversion Calculator
Calculate mg to mL with precision
The calculations are for conversion assistance only. Dosing may vary from patient to patient and is based on your own medical judgment as a healthcare provider.
Oral liquids may help overcome certain barriers to treatment success
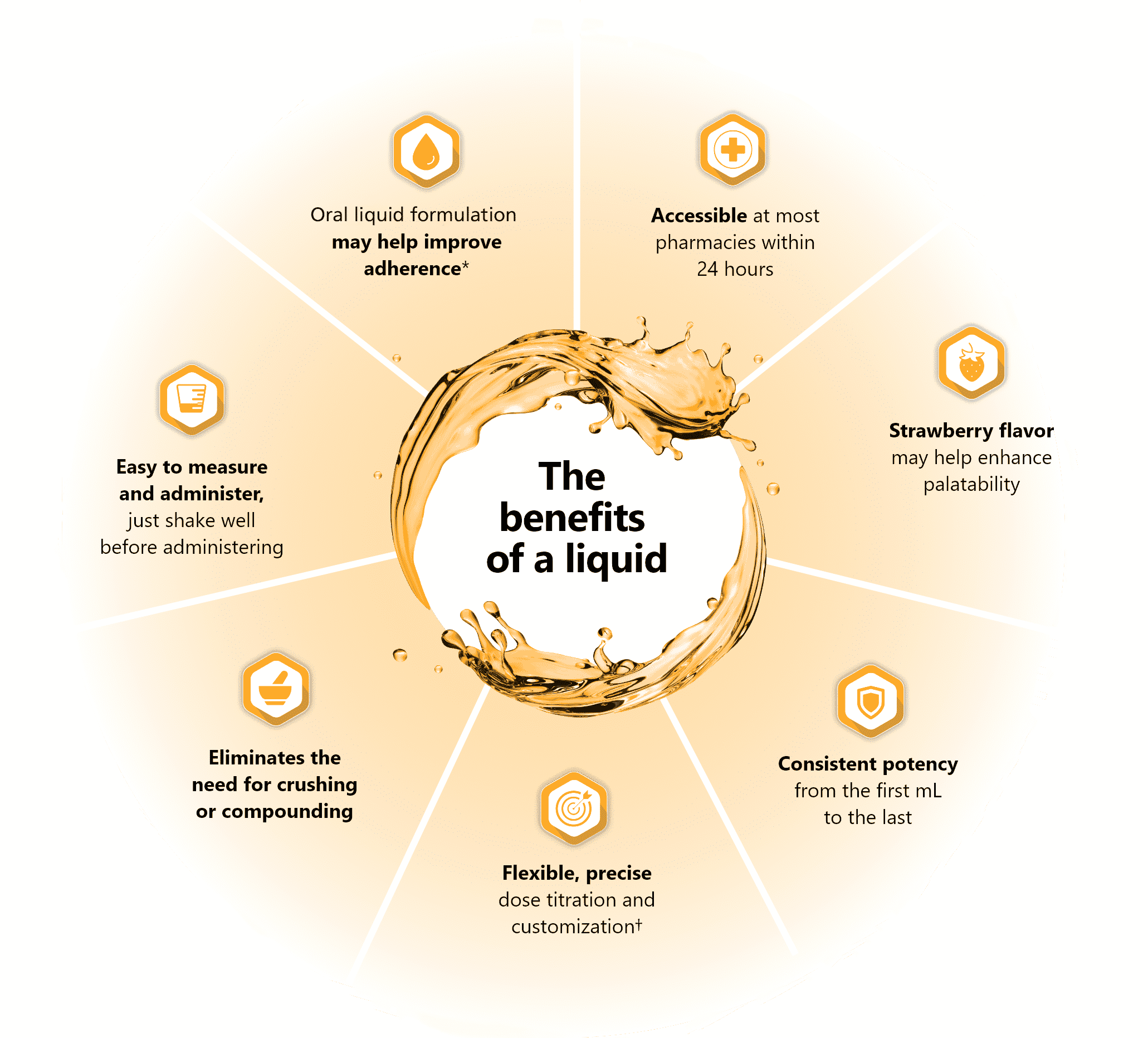
- *ZONISADE® does not have head-to-head data on adherence.
- †A calibrated measuring device is recommended to measure and deliver the prescribed dose accurately. A household teaspoon or tablespoon is not an adequate measuring device. Once opened, unused portion should be discarded after 30 days.2
Why Healthcare Providers Choose Zonisamide For Epilepsy
American Epilepsy Society Guidelines include zonisamide for use in treatment-resistant epilepsy based on Level A, B Recommendations3
- Level A recommendations are established as effective to reduce seizure frequency
- Level B recommendations should be considered to reduce seizure frequency
Clinical trials have shown zonisamide to be well tolerated with a good safety profile, which is supported by an excess of 2 million patient-years of clinical exposure4
Long half-life enables once-daily dosing2‡
- Patients who miss a dose should resume dosing at their next regularly scheduled dose — no makeup dose needed!
- ‡After a single-dose administration, renal clearance of zonisamide is approximately 3.5 mL/min. The clearance of an oral dose of zonisamide from red blood cells is 2 mL/min. The elimination half-life of zonisamide in plasma is approximately 63 hours.
Did you know?
4 out of 5
adults take several pills each day and nearly half report difficulty swallowing pills5
Pill dysphagia can result in low adherence and treatment failure6
Did you know?
Zonisamide powder can have a bitter taste and disliking taste was considered a barrier to clinical outcomes for adolescent epilepsy patients.7,8
Strategies to improve adherence include pleasant-tasting medications, liquid formulations, and simplified drug regimens (ideally once-daily regimens are preferred).9
Did you know?
Listed by NIOSH as a hazardous drug, zonisamide
presents an occupational hazard for those pregnant, breastfeeding, or trying to conceive.10
NIOSH = National Institute of Occupational Safety and Health
Ready-to-use medications eliminate the need for formulation manipulation, like compounding or crushing solid doses of zonisamide, reducing the risk of hazardous exposure.11
Provider Resources
From prescribing to dispensing, these expert insights and educational materials are here to help you stay informed
PUBLICATION
“Dysphagia in Epilepsy Patients: The Silent Enemy”
by Dr. James Wheless, et. al
ABSTRACT
“Matching Anti-Seizure Medications’ Formulations to Patients: Why It Matters!”
by Dr. James Wheless and MyHealthTeam

Epilepsy Support Organizations
These communities and groups may be able to support you and your patients
Coverage and Assistance Information
Explore our downloadable support materials designed to help simplify patient access
Looking to learn more?
Fill out the form below with your question or request, and an Azurity representative will respond as soon as possible.
Important Safety Information
ZONISADE® (zonisamide oral suspension), 100 mg/5 mL
ZONISADE is indicated as adjunctive therapy for the treatment of partial-onset seizures in adults and pediatric patients 16 years of age and older.
Inform patients that a calibrated measuring device is recommended to measure and deliver the prescribed dose accurately. A household teaspoon or tablespoon is not an adequate measuring device.
ADDITIONAL IMPORTANT SAFETY INFORMATION
Contraindications
Known hypersensitivity to sulfonamides, zonisamide, or any formulation ingredients.
Warnings and Precautions
Potentially fatal reaction to Sulfonamides: Fatalities have occurred as a result of severe reactions to sulfonamides (ZONISADE is a sulfonamide) including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias. If signs of hypersensitivity or other serious reactions occur, discontinue ZONISADE immediately.
Serious Skin Reaction: Serious skin reactions (Stevens-Johnson syndrome and toxic epidermal necrolysis) have been reported. Consideration should be given to discontinuing ZONISADE in patients who develop an otherwise unexplained rash. If the drug is not discontinued, patients should be observed frequently. Inform patients about the signs of serious skin reactions.
Serious Hematologic Events: Aplastic anemia and agranulocytosis have been reported in patients who received zonisamide treatment. There is inadequate information to assess the relationship, if any, between dose and duration of treatment and these events.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multi-Organ Hypersensitivity: Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multi-organ hypersensitivity, has occurred with zonisamide. Some of these events have been fatal or life-threatening. If signs or symptoms of DRESS are present, the patient should be evaluated immediately. ZONISADE should be discontinued if an alternative etiology for the signs or symptoms cannot be established.
Oligohidrosis and Hyperthermia in Pediatric Patients: ZONISADE is not approved for use in pediatric patients below 16 years of age. Pediatric patients appear to be at an increased risk for zonisamide-associated oligohidrosis and hyperthermia. Patients, especially pediatric patients, treated with ZONISADE should be monitored closely for evidence of decreased sweating and increased body temperature, especially in warm or hot weather. Caution should be used when ZONISADE is prescribed with other drugs that predispose patients to heat-related disorders; these drugs include but are not limited to, carbonic anhydrase inhibitors and drugs with anticholinergic activity.
Acute Myopia and Secondary Angle Closure Glaucoma: Acute myopia and secondary angle closure glaucoma have been reported in patients receiving zonisamide. Elevated intraocular pressure can lead to serious sequelae, including permanent vision loss if left untreated. Symptoms typically occur within one month after initiating zonisamide therapy. The primary treatment to reverse symptoms is the discontinuation of zonisamide. Myopia and secondary angle closure glaucoma usually resolve or improve after discontinuation of zonisamide.
Suicidal Behavior and Ideation: Antiepileptic drugs (AEDs), including ZONISADE, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Metabolic Acidosis: Metabolic acidosis was commonly observed in adults and pediatric patients in clinical trials and is caused by renal bicarbonate loss due to the inhibitory effect of zonisamide on carbonic anhydrase. Pediatric patients may be more likely to develop metabolic acidosis than adults. Conditions or therapies that predispose to acidosis (such as renal disease, severe respiratory disorders, status epilepticus, diarrhea, ketogenic diet, or specific drugs) may be additive to the bicarbonate lowering effects of zonisamide. Measurement of baseline and periodic serum bicarbonate during treatment is recommended. If metabolic acidosis develops, consideration should be given to either dose reduction or discontinuation of therapy using dose tapering.
Seizures on Withdrawal: As with other AEDs, abrupt withdrawal of ZONISADE in patients with epilepsy may precipitate increased seizure frequency or status epilepticus. Dose reduction or discontinuation of ZONISADE should be done gradually.
Teratogenicity: ZONISADE may cause fetal harm when administered to pregnant women and should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Women of childbearing potential who are given ZONISADE should be advised to use effective contraception.
Cognitive/Neuropsychiatric Adverse Reactions: Use of zonisamide was frequently associated with central nervous system-related adverse reactions including psychiatric symptoms, cognitive dysfunction, and somnolence or fatigue.
Hyperammonemia and Encephalopathy: Hyperammonemia and encephalopathy have been reported with the postmarketing use of zonisamide. The risks may be increased in patients treated with zonisamide and concomitantly taking valproic acid or topiramate. Measure serum ammonia concentration if signs or symptoms of encephalopathy occur. Hyperammonemia resulting from zonisamide resolves when zonisamide is discontinued. Hyperammonemia from zonisamide may resolve or decrease in severity with a decrease of the daily dose.
Kidney Stones: ZONISADE may increase the risk for kidney stones. Instruct patients to stay well hydrated while taking ZONISADE.
Effect on Renal Function: ZONISADE can cause an increase in serum creatinine and blood urea nitrogen (BUN). ZONISADE should be discontinued in patients who develop acute renal failure or a clinically significant sustained increase in the creatinine/BUN concentration. ZONISADE should not be used in patients with renal failure (estimated [GFR] < 50 mL/min) as there has been insufficient experience concerning drug dosing and toxicity.
Status Epilepticus: Status epilepticus has been reported at a rate of 1% across all controlled and uncontrolled epilepsy studies in patients treated with zonisamide.
Laboratory Tests: ZONISADE may increase serum chloride and alkaline phosphatase, and decrease serum bicarbonate, phosphorus, calcium, and albumin. Clinical management should include a periodic assessment of laboratory values.
Adverse Reactions
The most common adverse reactions with ZONISADE (an incidence at least 4% greater than placebo) in controlled clinical trials and shown in descending order of frequency were somnolence, anorexia, dizziness, ataxia, agitation/irritability, and difficulty with memory and/or concentration.
Drug Interactions
ZONISADE should be used with caution if used in combination with alcohol or other CNS depressants.
Concomitant use of ZONISADE with any other carbonic anhydrase inhibitor may increase the severity of metabolic acidosis and may also increase the risk of kidney stone formation.
USE IN SPECIFIC POPULATIONS
Pregnancy
ZONISADE may cause serious adverse fetal effects, based on clinical and nonclinical data.
Advise pregnant patients to enroll in the NAAED Pregnancy Registry. This can be done by calling the toll-free number 1-888-233-2334 and must be done by patients themselves.
Lactation
Zonisamide is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from ZONISADE, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Female and Males of Reproductive Potential
Based on animal data, ZONISADE can cause fetal harm when administered to a pregnant woman, and female fertility may be compromised with ZONISADE.
Pediatric Use
The safety and effectiveness of ZONISADE in pediatric patients below the age of 16 have not been established.
The Important Safety Information does not include all the information needed to use ZONISADE safely and effectively. For additional safety information, please see the accompanying full Prescribing Information for ZONISADE.
To report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals, Inc. at 1-800-461-7449, or FDA at 1-800-FDA-1088 or www.fda.gov/MedWatch.
References
- 1. Orange Book: approved drug products with therapeutic equivalence evaluations. Accessed May 15, 2025. https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm
- 2. ZONISADE [package insert]. Woburn, MA: Azurity Pharmaceuticals, Inc.; 2023.
- 3. Kanner AM, Ashman E, Gloss D, et al. Practice guideline update summary: efficacy and tolerability of the new antiepileptic drugs I: treatment of new-onset epilepsy: report of the guideline development, dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2018;91(2):74-81. doi:10.1212/WNL.0000000000005755
- 4. Sills G, Brodie M. Pharmacokinetics and drug interactions with zonisamide. Epilepsia. 2007;48(3):435-441. doi:10.1111/j.1528-1167.2007.00983.s
- 5. Fields J, Go JT, Schulze KS. Pill Properties that cause dysphagia and treatment failure. Curr Ther Res Clin Exp. 2015;77:79-82. doi:10.1016/j.curtheres.2015.08.002
- 6. Liu F, Ghaffur A, Bains J, Hamdy S. Acceptability of oral solid medicines in older adults with and without dysphagia: a nested pilot validation questionnaire based observational study. Int J Pharm. 2016;512(2):374-381. doi:10.1016/j.ijpharm.2016.03.007
- 7. Masuda Y, Ishizaki M, Shimizu M. Zonisamide: Pharmacology and clinical efficacy in epilepsy. CNS Drug Reviews. 1998;4(4):341–360. https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1527-3458.1998.tb00075.x
- 8. Gutierrez-Colina AM, Smith AW, Mara CA, Modi AC. Adherence barriers in pediatric epilepsy: from toddlers to young adults. Epilepsy Behav. 2018;80:229-234. doi:10.1016/j.yebeh.2018.01.031
- 9. Chappell F. Medication adherence in children remains a challenge. Prescriber. 2015;26(12):31-34.
- 10. NIOSH list of hazardous drugs in Healthcare Settings, 2024. Accessed May 8, 2025. https://www.cdc.gov/niosh/docs/2025-103/default.html
- 11. Duke University Occupational & Environmental Safety Office. (March 2025). Safe handling of hazardous drugs. https://www.safety.duke.edu/sites/default/files/V-HazardousDrugs.pdf. Accessed June 24, 2025




